College Level Sciences

- Molecular Biology: This discipline discusses the molecular systems of DNA replication, repair, transcription, translation, sequencing, protein synthesis, and gene regulation in various organisms. Essentially it is how molecules, nucleic acids, and proteins work together within the cell to promote correct development, growth, and division.
- Cell Biology: As the name suggests, cell biology focuses on the structuring, synthesis, and functions of prokaryotic and eukaryotic cells. Cells are the basis of living and thus it is essential to study their life cycle, physiological properties and organelles, environmental interactions, and death.
- Physiology: Physiology includes both the chemical and physical processes of living organisms. Typically, most physiology courses are attentive to the human body and its systems: nervous, endocrine, muscle, skeletal, circulatory, lymphatic, immune, digestive, excretory and reproductive.
- Biochemistry: This area investigates the chemistry of living matter. This includes the properties, relationships, and processes of metabolism, proteins, and enzymes. In regards to metabolism its cellular components include carbohydrates, proteins, lipids and nucleic acids.
- Ecology: Ecology deals with the relationship and exchanges of organisms and their biotic and abiotic environments. A number of environmental variables are considered when talking about ecology they are: composition, distribution, biomass, number, and changing states of organisms within and among ecosystems.
- General: Gen chem. (as its commonly known as) provides an overview on different topics dealing with chemistry. Topics include the atomic theory, chemical bonding, fundamentals of physical chemistry, prediction of reaction products, thermodynamics, nuclear chemistry, electrochemistry, chemical kinetics, and stoichiometry.
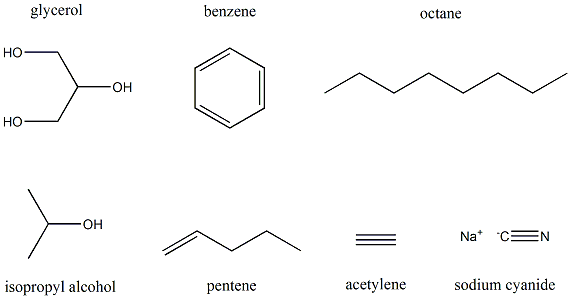
- Organic: O-chem examines the structure, properties, composition, reactions, and preparation (by synthesis or by other means) of alkenes, ethers, alcohol, alkyl halides alkanes, methane, and benzene, hybrid orbitals, polarity of bonds and molecules, hydrocarbons, and their derivatives, halogenation, and combustion.
- Physical: is predominantly (but not always) a macroscopic or supra-molecular science, as the majority of the principles on which physical chemistry was founded are concepts related to the bulk. It studies the macroscopic, atomic, subatomic, and particulate phenomena in chemical systems in terms of physical laws and concepts.
- Physics: In intro courses undergrads are introduced to mechanics, kinematics, vectors, wave phenomena, fluids, circuit elements and analysis, electricity and magnetism, optics, diffusion, and heat flow.
|



